舒泰神(300204.SZ)三大新藥項目在行動 聚焦重症COVID-19的有效治療
新華社北京10月19日電 中共中央政治局委員、國務院副總理孫春蘭19日到國家藥監局藥品審評中心調研,瞭解新冠病毒藥物臨牀試驗、審評服務等情況,並召開相關企業和專家座談會,聽取藥物研發工作的意見建議。孫春蘭強調,要深入貫徹習近平總書記關於疫情防控和藥品研發的重要指示精神,落實黨中央、國務院決策部署,尊重科學、遵循規律,集中科研力量,全力開展新冠治療藥物研發攻關,力爭早日取得突破,為科學防控疫情、保障人民健康提供有力支撐。
孫春蘭強調,要組織專家對現有研發藥品進行認真遴選,分類給予支持和指導,對於臨牀數據完整的,及時推動審批上市;對作用機制明確、潛在療效明顯、安全性好的,及早介入支持開展臨牀試驗。要統籌做好境內外臨牀試驗,加強境內臨牀研究平台建設和臨牀資源有效利用,引導企業有序做好境外臨牀研究,加快試驗進度。
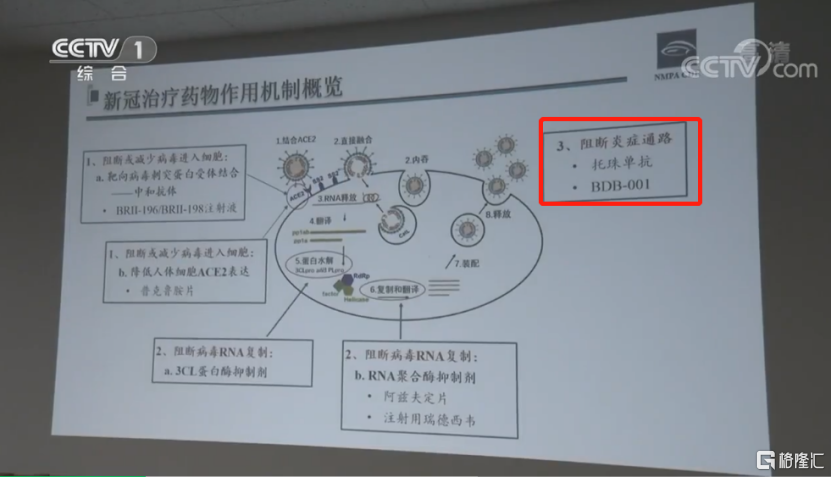
細胞因子風暴和COVID-19
在許多炎症情況下,例如骨關節炎、類風濕性關節炎和最近的新冠病毒肺炎,當免疫系統過度和不可控制地被激活後,會出現主要的併發症和廣泛的組織損傷。尋找新的方法來選擇性地控制這種過度活動可能會有重大的臨牀益處。
細胞因子風暴又稱炎症因子風暴,在高致病性病毒(如甲型流感病毒、SARS、MERS、SARS-CoV-2)感染後,通過過度激活補體系統誘發。細胞因子風暴產生後,過度激活的免疫系統會對人體進行攻擊,從而導致急性肺損傷、呼吸窘迫綜合徵等嚴重臨牀後果,進而危及患者生命。
BDB-001是針對人C5a靶點的特異性單克隆抗體,可高效、特異地抑制補體系統的過度激活,從源頭上有效防止細胞因子風暴的產生,從而減輕因感染性疾病造成的危重症傷害。
BDB-001作為舒泰神的重點研發項目,當下正在全球推進臨牀II/Ⅲ期試驗,截止日前全球入組例數超過95%。針對C5a靶點的近千例臨牀用藥也對該藥物的安全性做出了比較充分的驗證。
舒泰神在通過對BDB-001所針對的C5a靶點的多年研究及國際化臨牀趨勢的跟蹤後,在感染類疾病引起的細胞因子風暴過程中增加了同靶點STSA-1002的中美雙報,目前已獲得中美兩國臨牀試驗許可並在美國完成首例受試者給藥。
舒泰神的STSA-1005注射液是人GMR通路的特異性強效拮抗劑。它在體外和體內都顯示出對GM-CSF/GMR信號通路的顯著抑制作用。基於公開研究顯示,阻斷GM-CSF/GMR的生物活性可以下調促炎反應,並可能為重症COVID-19肺炎患者提供臨牀益處。
舒泰神三個治療性一類新藥均針對新冠重症人羣,為感染後的重症治療提供有力武器,希望不同作用機制、不同技術路線的必需、急需治療藥物守護患者,更快更好地服務於人民羣眾的生命安全和身體健康!
來源:中國要聞、舒泰神公司微信公眾號
Follow us
Find us on
Facebook,
Twitter ,
Instagram, and
YouTube or frequent updates on all things investing.Have a financial topic you would like to discuss? Head over to the
uSMART Community to share your thoughts and insights about the market! Click the picture below to download and explore uSMART app!

Disclaimers
uSmart Securities Limited (“uSmart”) is based on its internal research and public third party information in preparation of this article. Although uSmart uses its best endeavours to ensure the content of this article is accurate, uSmart does not guarantee the accuracy, timeliness or completeness of the information of this article and is not responsible for any views/opinions/comments in this article. Opinions, forecasts and estimations reflect uSmart’s assessment as of the date of this article and are subject to change. uSmart has no obligation to notify you or anyone of any such changes. You must make independent analysis and judgment on any matters involved in this article. uSmart and any directors, officers, employees or agents of uSmart will not be liable for any loss or damage suffered by any person in reliance on any representation or omission in the content of this article. The content of the article is for reference only and does not constitute any offer, solicitation, recommendation, opinion or guarantee of any securities, virtual assets, financial products or instruments. Regulatory authorities may restrict the trading of virtual asset-related ETFs to only investors who meet specified requirements. Any calculations or images in the article are for illustrative purposes only.
Investment involves risks and the value and income from securities may rise or fall. Past performance is not indicative of future performance. Please carefully consider your personal risk tolerance, and consult independent professional advice if necessary.





