凱普生物(300639.SZ):全資子公司獲得韓國、日本發明專利授權通知書
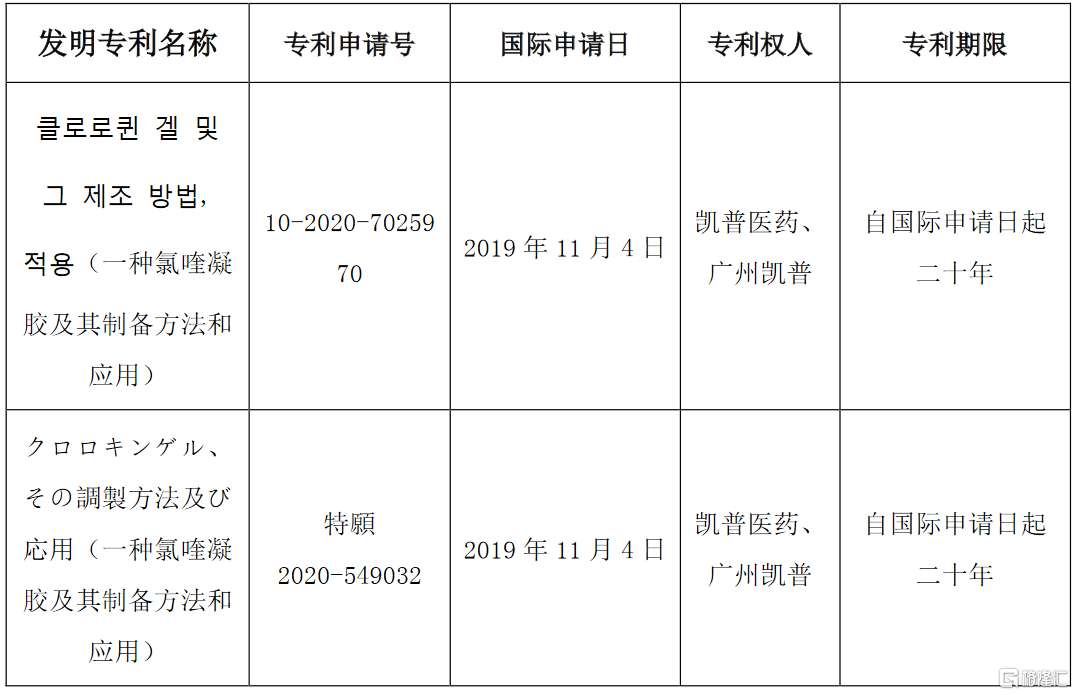
格隆匯5月19日丨凱普生物(300639.SZ)公佈,公司的全資子公司廣州凱普醫藥科技有限公司(“凱普醫藥”)、廣州凱普生物科技有限公司(“廣州凱普”)於2019年11月22日獲國家發明專利授權,專利名稱:一種氯喹凝膠及其製備方法和應用,授權號:ZL 201811474701.3,詳見公司2019年10月23日發佈的《關於全資子公司獲得授予發明專利權通知書的公吿》(公吿編號:2019-071)。
近日,凱普醫藥及廣州凱普收到Korean Intellectual Property Office(韓國知識產權局)和Japan Patent Office(日本特許廳)的發明專利授權通知書,具體情況如下:
該發明屬於生物醫藥技術領域,涉及一種氯喹凝膠的製備及應用。該發明提供了一種氯喹納米微球的製備,含有水溶性納米微球載體、以及氯喹或氯喹衍生物,氯喹或氯喹衍生物與水溶性納米微球載體的質量比不超過1:3。本發明將水溶性納米微球載體與氯喹或氯喹衍生物聯合制備成納米微球,有效解決了氯喹的副作用和抗藥性問題;可延長藥物在治療部位的滯留時間,提高了藥物的生物利用度和治療效果,能有效治療外生殖器感染性疾病,顯著增加療效;不僅拓寬氯喹的用途,拓寬其應用方案,並且其製備方法簡便、易於合成,無毒副作用,安全性好,可進行新藥的產業化應用。
公司已運用該發明技術開發藥品“磷酸氯喹凝膠”,目前正在開展II期有效性及安全性評價的臨牀試驗,目前試驗進展順利。“磷酸氯喹凝膠”適應症為皮膚外用治療HPV病毒感染引起的各種皮膚疣、外生殖器及肛周尖鋭濕疣,為國內外尚未批准的新適應症。通過皮膚外用給藥,減少全身藥物暴露量,提高藥品的有效性和安全性。本藥品自2014年開始研發,於2018年3月獲國家藥品監督管理局頒發《藥物臨牀試驗批件》,開展I期臨牀試驗。2019年12月4日,廣州凱普正式向CDE提交II期臨牀試驗方案。2020年2月21日,公司收到CDE回覆同意本項目開展II期臨牀試驗。
此次國外發明的取得將有利於加強公司自主知識產權的國際保護並進一步完善公司的知識產權體系,充分發揮公司的知識產權優勢,促進公司產品結構的不斷豐富,提升公司的核心競爭力,不會對公司目前的經營狀況產生重大的影響。
公司將嚴格依照辦理登記手續通知書的內容辦理登記手續,在按期辦理登記手續費後,韓國知識產權局及日本特許廳將頒發發明專利證書,並予以登記和公吿。專利權自公吿之日起生效,有效期自國際申請日起二十年。
Follow us
Find us on
Facebook,
Twitter ,
Instagram, and
YouTube or frequent updates on all things investing.Have a financial topic you would like to discuss? Head over to the
uSMART Community to share your thoughts and insights about the market! Click the picture below to download and explore uSMART app!

Disclaimers
uSmart Securities Limited (“uSmart”) is based on its internal research and public third party information in preparation of this article. Although uSmart uses its best endeavours to ensure the content of this article is accurate, uSmart does not guarantee the accuracy, timeliness or completeness of the information of this article and is not responsible for any views/opinions/comments in this article. Opinions, forecasts and estimations reflect uSmart’s assessment as of the date of this article and are subject to change. uSmart has no obligation to notify you or anyone of any such changes. You must make independent analysis and judgment on any matters involved in this article. uSmart and any directors, officers, employees or agents of uSmart will not be liable for any loss or damage suffered by any person in reliance on any representation or omission in the content of this article. The content of the article is for reference only and does not constitute any offer, solicitation, recommendation, opinion or guarantee of any securities, virtual assets, financial products or instruments. Regulatory authorities may restrict the trading of virtual asset-related ETFs to only investors who meet specified requirements. Any calculations or images in the article are for illustrative purposes only.
Investment involves risks and the value and income from securities may rise or fall. Past performance is not indicative of future performance. Please carefully consider your personal risk tolerance, and consult independent professional advice if necessary.




