uSMART盈立智投11月9日消息,德琪醫藥-B今起招股,一手入場費9131.09港元,高盛、摩根大通聯席保薦。
招股信息:
(1)簡稱及代碼: 德琪醫藥-B,6996.HK
(2)招股日期: 11月9日~12日
預期公佈中籤日期:11月19日
上市日期: 11月20日
計息日: 7天
(3)發行價格: 15.8港元~18.08港元,每手股數500股
(4)入場費: 9,131.09港元,乙頭需認購30萬股(600手),金額約547.86萬港元;以3.5%年利率和10倍槓桿計算,乙頭利息約3310港元港元

(5)發行股數: 154,153,500股,公開發售佔比10%,國際發售佔比90%
(6)超額配股權: 有,可按發售價發行最多2312.3萬股(佔發售股份的15%),以補足國配的超額認購
(7)集資金額: 24.36億港元~27.87億港元
(8)市值: 105.58億港元~120.81億港元
(10)保薦人及近兩年IPO首日表現:
高盛,12勝4負,最佳項目爲歐康維視生物-B(首日收漲152.39%)
摩根大通,8勝2負,最佳項目爲康方生物-B(首日收漲50.19%)
(11)穩定價格操作人: 高盛
(12)包銷商:7家,包銷傭金3%+獎金1%
(13)基石投資者: 按發售價中位數計,10名基石合共鎖定53.25%的發售股份,禁售期6個月
富達:認購32,027,500股,佔發售股份的20.78%
新加坡主權財富基金GIC:認購9,150,500股,佔發售股份的5.94%
貝萊德:認購6,863,000股,佔發售股份的4.45%
博裕資本:認購6,863,000股,佔發售股份的4.45%
Cormorant:認購6,863,000股,佔發售股份的4.45%
高瓴資本:認購6,863,000股,佔發售股份的4.45%
Sequoia Capital China Growth:認購6,863,000股,佔發售股份的4.45%
CRF(中國國新):認購2,196,000股,佔發售股份的1.42%
Laurion Capital:認購2,196,000股,佔發售股份的1.42%
Octagon Investments:認購2,196,000股,佔發售股份的1.42%

(14)首次公開發售前投資者:
自本公司成立以來,已與首次公開發售前投資者(包括富達、BlackRock、GIC、高瓴、博裕資本、方源資本及啓明創投)訂立幾輪融資協議。
首次公開發售前投資者的廣泛及多元化羣體包括專注於生物製藥及╱或更廣泛醫療保健行業的資深投資者及戰略投資者(包括Celgene、藥明康德及泰格醫藥)。
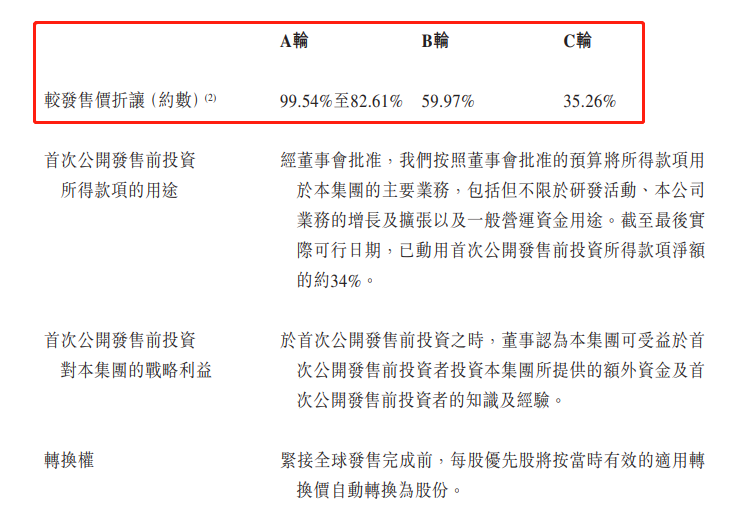
A輪成本較發售價折讓99.54%-82.61%
B輪成本較發售價折讓59.97%
C輪成本較發售價折讓35.26%

2、回撥機制
1)公開發售認購15倍以內,公開發售股份比例10%,甲乙組共有30832手。
2)公開發售認購15-50倍時,公開發售股份比例回撥至30%,甲乙組共有92492手。
3)公開發售認購50-100倍時,公開發售股份比例回撥至回撥40%,甲乙組共有123323手。
4)公開發售認購超過100倍時,公開發售股份比例回撥至回撥50%,甲乙組共有154154手。
3、股東陣容
德琪醫藥的股東陣容異常耀眼,醫藥行業的戰略投資者包括百時美施貴寶旗下的新基中國、藥明康德和泰格醫藥,國內外頂尖機構投資者包括富達、貝萊德、GIC、高瓴、博裕資本、方源資本、啓明創投及泰康資管。
公司業務
截至最後實際可行日期,公司已戰略性地設計並組建起一條擁有12款腫瘤藥物資產的創新型研發管線,包括兩種晚期臨牀產品、四種早期臨牀產品和六種臨牀前產品。
截至同日,公司有九項正在進行的臨牀試驗(包括三項研究者發起的試驗)及八項計劃啓動的臨牀試驗,並已在亞太地區的多個司法管轄區取得九個IND批件。
同類首款及同類唯一SINE化合物在亞太地區具有重大短期商業化機會
ATG-010 (selinexor),或者公司的核心產品之一XPOVIO® (selinexor)是一款同類首款及同類唯一的抑制核輸出蛋白XPO1的SINE化合物,可促使腫瘤抑制蛋白在細胞核內積累,選擇性誘導癌細胞凋亡。
FDA已有條件加速批準XPOVIO® (selinexor)用於治療復發╱難治性多發性骨髓瘤(基於IIb期STORM試驗第二部分的結果)及復發╱難治性瀰漫大B細胞淋巴瘤(基於IIb期SADAL試驗的結果)。
FDA於2020年7月受理Karyopharm的補充NDA(基於驗證性III期BOSTON試驗的結果),作爲之前至少接受業務。
ATG-010 (selinexor)是首款及唯一一種獲FDA批準的可同時用於治療復發╱難治性多發性骨髓瘤及復發╱難治性瀰漫大B細胞淋巴瘤的藥物,亦是獲FDA批準的唯一一種治療復發╱難治性瀰漫大B細胞淋巴瘤的單藥口服療法。
打新有風險,申購需謹慎,詳情閱讀《德琪醫藥-B招股書》。



