中國生物製藥(01177.HK):派安普利上市獲藥監局受理,業績可期,維持“增持”評級
機構:國泰君安
評級:增持
本報告導讀:
公司公告抗 PD-1 單抗派安普利上市申請獲藥監局受理,申報適應症為復發或難治性經典型霍奇金淋巴瘤,公司產品線不斷創新升級,維持增持評級。
摘要:
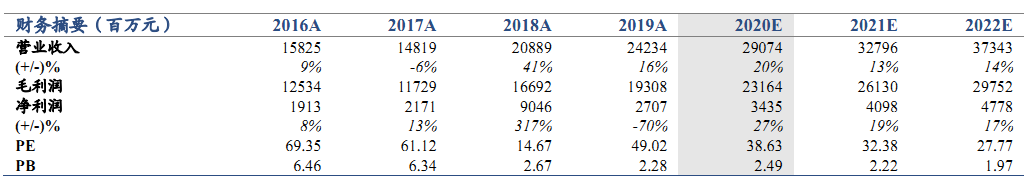
維持“增持”評級。公司2020 年5月26日發佈公告,其與康方生物共同開發及商業化的抗PD-1單抗藥物安尼可(通用名:派安普利,研發代號: AK105)的新藥上市申請,已獲中國國家藥品監督管理局受理,用於治療復發或難治性經典型霍奇金淋巴瘤患者。我們維持公司2020-2022年EPS預測值0.27/0.33/0.38元,考慮到公司大品種研發成果不斷落地,產品線不斷創新升級,上調目標價至13.80 港元(原為 12.13港元),維持增持評級。
派安普利可能是第5個上市的國產 PD-1抑制劑,有望藉助公司強大銷售網絡快速放量。截止2020年5月,我國PD-(L)1產品已體現出進口和國產4+4的局面,從上市申報適應症來看,派安普利延續了國產 PD-1用小適應症快速獲批上市的策略。國產和進口PD-1由於定價不同,搶佔的是不同消費層級的患者,派安普利未來的上市,更多對現有的國產PD-1產品有衝擊。我們預計卡瑞利珠單抗在 2019年上市5個月銷量突破10億,(對比信達和君實的 PD-1產品已在 2018年底獲批,而2019年全年銷售額分別為 10.2億、 7.8億),可以看出, PD-1的推廣與企業醫院終端覆蓋面、銷售體系等商業化綜合能力休慼相關。未來派安普利上市,有望憑藉公司 3000多人的腫瘤銷售網絡快速放量。
商業化策略逐漸清晰:以小適應症快速上市,大適應症的臨牀加快跟進。截止 2020 年 5 月 16 日,派安普利共有 13 項臨牀正在進行,其中 9項試驗同時在中美進行了申報。目前已經進入三期階段的有肺癌一線、胃癌二線和晚期肝細胞癌 3項患者基數較大的適應症,其中胃癌和肝癌的用藥方式皆為派安普利與安羅替尼聯用,“強強組合銷售+大適應症策略”的發展態勢已初見端倪。
催化劑:新產品及新適應症的研發進展,光腳品種的放量。
風險提示:帶量採購的風險;新藥研發的不確定性風險。
Follow us
Find us on
Facebook,
Twitter ,
Instagram, and
YouTube or frequent updates on all things investing.Have a financial topic you would like to discuss? Head over to the
uSMART Community to share your thoughts and insights about the market! Click the picture below to download and explore uSMART app!

Disclaimers
uSmart Securities Limited (“uSmart”) is based on its internal research and public third party information in preparation of this article. Although uSmart uses its best endeavours to ensure the content of this article is accurate, uSmart does not guarantee the accuracy, timeliness or completeness of the information of this article and is not responsible for any views/opinions/comments in this article. Opinions, forecasts and estimations reflect uSmart’s assessment as of the date of this article and are subject to change. uSmart has no obligation to notify you or anyone of any such changes. You must make independent analysis and judgment on any matters involved in this article. uSmart and any directors, officers, employees or agents of uSmart will not be liable for any loss or damage suffered by any person in reliance on any representation or omission in the content of this article. The content of the article is for reference only and does not constitute any offer, solicitation, recommendation, opinion or guarantee of any securities, virtual assets, financial products or instruments. Regulatory authorities may restrict the trading of virtual asset-related ETFs to only investors who meet specified requirements. Any calculations or images in the article are for illustrative purposes only.
Investment involves risks and the value and income from securities may rise or fall. Past performance is not indicative of future performance. Please carefully consider your personal risk tolerance, and consult independent professional advice if necessary.




