全球抗疫仍在緊張進行,又一款新冠候選疫苗獲准開始臨牀試驗。
週三,德國生物科技公司BioNTech獲得當地疫苗監管機構Paul Ehrlich Institut批准,可以開始進行其mRNA疫苗的人體臨牀試驗。這是歐洲首個獲批人體臨牀試驗的新冠候選疫苗。
BioNTech已成立12年,不過此前並未推出面向市場的產品。該公司1月開始進行新冠疫苗研發,投入的力量大約為400人,目前已在小鼠身上對較早版本的候選疫苗進行了試驗。
接下來的人體臨牀試驗一期將在200名健康測試者身上進行,二期測試會擴大覆蓋範圍,測試者將超過千人,納入有較高風險感染新冠病毒的羣體。
Paul Ehrlich Institut負責人Klaus Cichutek表示,一期人體臨牀試驗可能要花費三個月到五個月時間。
受臨牀試驗獲批影響,在納斯達克上市的BioNTech股票週三盤前一度大漲45%,當天收盤上漲26.6%。

此前,BioNTech曾表示,它與美國製藥公司輝瑞在共同研發mRNA疫苗BNT162,這是BioNTech針對新冠疫情的“光速(Lightspeed)”項目中的第一種候選疫苗。
除了在德國之外,BioNTech的候選疫苗還將在海外測試。在美國,目前在等待監管批准。在中國,BioNTech已與復星醫藥達成合作,計劃開展BNT162的臨牀試驗。
3月16日,復星醫藥公告稱,公司控股子公司復星醫藥產業與BioNTech簽訂《許可協議》,BioNTech授權復星醫藥產業在區域內獨家開發、商業化基於其專有的mRNA技術平台研發的、針對新冠肺炎的疫苗產品。
公告還提到,復星醫藥產業將根據約定向BioNTech支付至多8500萬美元的許可費(包括首付款、臨牀開發註冊及銷售里程碑款項),並在約定的銷售提成期間內按該產品年度毛利的35%支付銷售提成。
在復星醫藥2019年業績溝通會上,復星醫藥總裁兼首席執行官吳以芳表示:“要徹底戰勝新冠疫情,還是需要靠疫苗。”他透露,復星醫藥希望與BioNTech、BioNTech的國外合作方以及國家藥監局一起努力,讓mRNA疫苗開發進度跟上國際腳步。
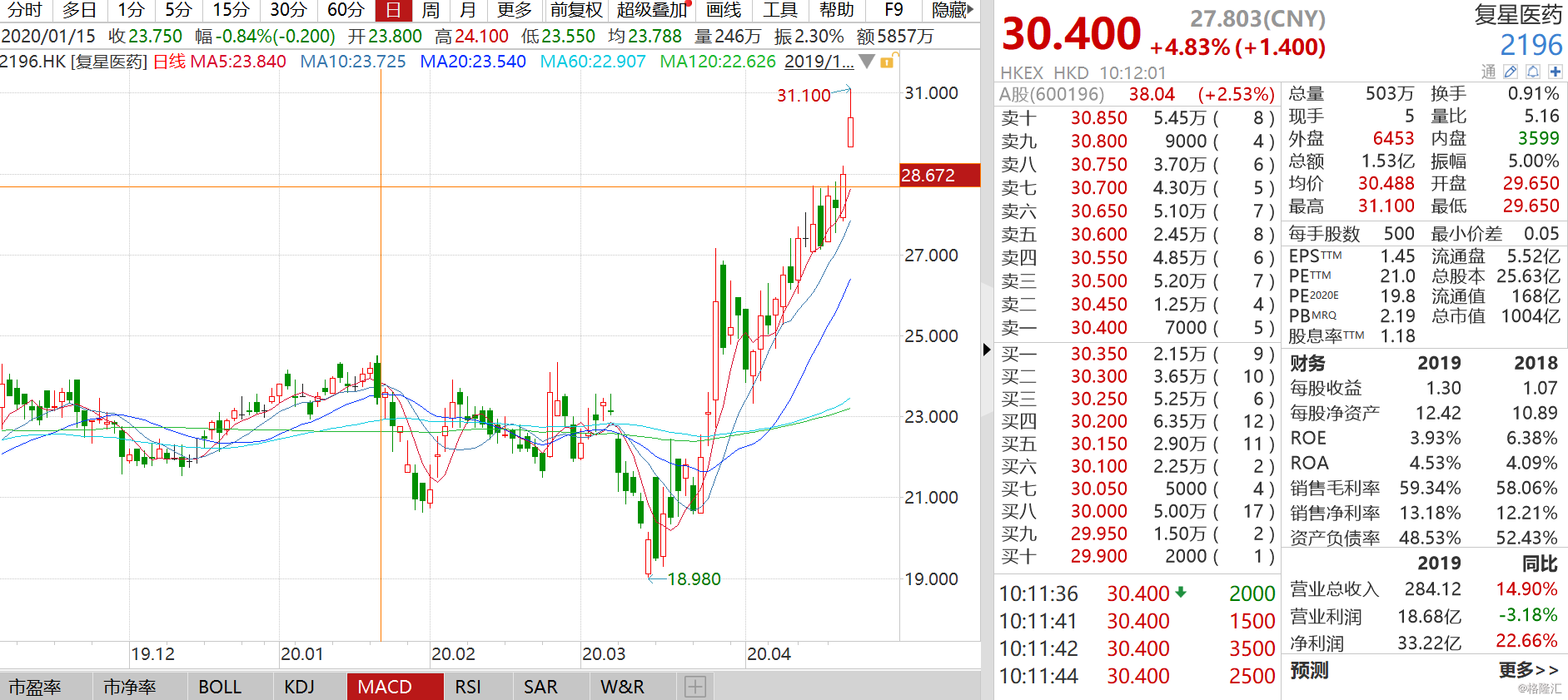
受此,復星醫藥股價持續上漲,截至發稿,漲近5%,報30.4港元,成交額達1.54億。
據世界衞生組織數據,此前全球共有70種新冠病毒疫苗正在研發中,有五款疫苗已經進入臨牀試驗,分別為中國的三款疫苗和美國的兩款疫苗。



