華大基因(300676.SZ)就新型冠狀病毒核酸檢測試劑盒產品已啟動FDA的EUA申報
格隆匯3月18日丨華大基因(300676.SZ)公佈,公司就新型冠狀病毒核酸檢測試劑盒產品已啟動美國食品藥品監督管理局(英文全稱“Food and Drug Administration”,“FDA”)的EUA申報,目前Pre-EUA已被正式受理。根據美國近期發佈的新型冠狀病毒肺炎相關檢測政策指南,公司將正式啟動該產品在美國臨牀市場的商業銷售。
產品基本信息如下:
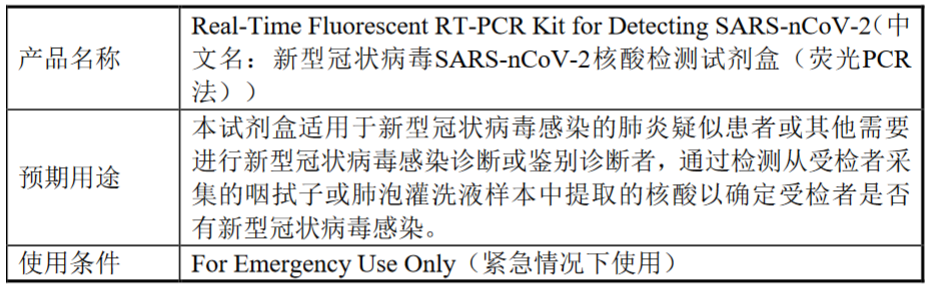
根據美國《聯邦食品、藥物和化粧品法案》第564條,在緊急情況下,當現有市場的產品無法滿足需求時,FDA官員可以允許,將未經FDA審批的醫療產品或現有醫療產品中未審批的新應用,用於診斷、治療、預防威脅到生命的疾病或情況。通常情況下,緊急使用授權(英文全稱“EmergencyUse Authorization”,“EUA”)的申報分為兩個階段,緊急使用預授權(英文全稱“Pre-EUA”)和緊急使用授權(英文全稱“EUA”),目前公司已向FDA遞交了Pre-EUA,該申報已於近日獲得受理,尚在審核過程中,後續還需要FDA的進一步反饋與認同,才能獲批EUA。
根據目前新型冠狀病毒在美國的蔓延情況,FDA於當地時間2020年3月16日發佈《新型冠狀病毒肺炎在公共危機下的診斷檢測政策指南》(英文全稱“Policy for Diagnostic Tests for Coronavirus Disease-2019 during the Public Health Emergency”,以下簡稱“《指南》”),根據《指南》相關內容顯示,符合條件的產品可以面向臨牀市場進行商業銷售。在《指南》條款下,公司已向FDA報備產品用途等相關信息,並提交銷售意願,在基於現有的方法學驗證基礎上,FDA已接受公司的產品申報,並允許公司在EUA獲批前,即自2020年3月17日起,在美國臨牀市場銷售其新型冠狀病毒SARS-nCoV-2核酸檢測試劑盒(熒光PRC法)產品。在緊急情況有效期內,公司上述檢測產品符合《指南》所述的商業銷售條件。如果在緊急情況終止後,公司仍希望在美國臨牀市場銷售該產品,則需要另行向FDA提交產品註冊申請。
截至公告披露日,根據美國FDA官網信息顯示,已有8家國外企業或機構的新型冠狀病毒檢測產品獲得了美國FDA的緊急使用授權。
此前,公司該檢測產品已取得了中國國家藥品監督管理局頒發的醫療器械注冊證,完成了歐盟CE認證,並獲得了歐盟自由銷售證書,相關內容詳見之前公告。
目前公司的新型冠狀病毒檢測產品處於EUA申報階段,按照美國現行的《指南》,上述檢測產品符合在美國臨牀市場進行商業銷售的條件。FDA EUA尚在審核過程中,公司正在按照流程積極推進FDA EUA的申報工作。
Follow us
Find us on
Facebook,
Twitter ,
Instagram, and
YouTube or frequent updates on all things investing.Have a financial topic you would like to discuss? Head over to the
uSMART Community to share your thoughts and insights about the market! Click the picture below to download and explore uSMART app!

Disclaimers
uSmart Securities Limited (“uSmart”) is based on its internal research and public third party information in preparation of this article. Although uSmart uses its best endeavours to ensure the content of this article is accurate, uSmart does not guarantee the accuracy, timeliness or completeness of the information of this article and is not responsible for any views/opinions/comments in this article. Opinions, forecasts and estimations reflect uSmart’s assessment as of the date of this article and are subject to change. uSmart has no obligation to notify you or anyone of any such changes. You must make independent analysis and judgment on any matters involved in this article. uSmart and any directors, officers, employees or agents of uSmart will not be liable for any loss or damage suffered by any person in reliance on any representation or omission in the content of this article. The content of the article is for reference only and does not constitute any offer, solicitation, recommendation, opinion or guarantee of any securities, virtual assets, financial products or instruments. Regulatory authorities may restrict the trading of virtual asset-related ETFs to only investors who meet specified requirements. Any calculations or images in the article are for illustrative purposes only.
Investment involves risks and the value and income from securities may rise or fall. Past performance is not indicative of future performance. Please carefully consider your personal risk tolerance, and consult independent professional advice if necessary.




