格隆匯8月16日丨貴州百靈(002424.SZ)公佈,公司於2015年1月26日與香港大學簽訂了《合作研究合同》,公司委託香港大學研究開發“糖寧通絡膠囊治療糖尿病及併發症作用機理的研究”項目。
近日公司收到香港大學關於《TNTL有效部位及其化學衍生物(BL03)研究紀要(第二次)》。現將相關情況公告如下:
一、糖寧通絡(TNTL)論文進展
1、TNTL在肥胖和糖尿病上的整體效應和作用機制:經過連續四年多的艱苦研究,一篇論文被世界著名雜誌《科學》系列雜誌《科學進展》正式接受,並於北京時間八月十五日(美國時間八月十四日)正式出版:
Science Advance科學進展
SBP2 deficiency in adipose tissue macrophage drive insulin resistance in obesity脂肪組織巨噬細胞中的SBP2缺乏驅動肥胖症中的胰島素抗性(影響因子12.804):
肥胖與糖尿病、心腦血管病以及惡性腫瘤的發生發展也密切相關。促炎活化和脂肪組織巨噬細胞(ATM)的積累與增加肥胖症患胰島素抵抗的風險關係密切。在這裏,我們描述了硒代半胱氨酸插入序列結合蛋白質2(SBP2)在維持肥胖的胰島素的敏感性的新作用。SBP2在飲食誘導的肥胖的小鼠ATM中受到抑制並與脂肪組織炎症相關。SBP2的丟失引發了ATM的代謝激活,誘導細胞內活性氧含量和炎性體,隨後促進IL1bata相關的局部增殖和促炎性巨噬細胞的浸潤。敲除特定ATM的肥胖小鼠中的SBP2,可通過增加脂肪組織炎症和擴張來促進胰島素抵抗。再表達SBP2改善胰島素敏感性。最後,一種特異性誘導ATM中SBP2表達的草藥配方TNTL可以通過實驗改善胰島素敏感性臨牀觀察顯示其改善了高血糖症糖尿病患者。該研究將ATM中的SBP2鑑定為治療肥胖症中胰島素抵抗的新靶點,對藥物開發及評價現有藥物十分關鍵,意義重大。
2、TNTL在糖尿病眼病上的效果和作用機制
Cell Communication and Signaling 細胞通訊與信號
OMICs Approache-Assisted Identification of Macrophage-Derived MIP-1γas the Therapeutic Target of Botanical Products TNTL in Diabetic Retinopathy用組學方法輔助鑑定巨噬細胞衍生的MIP-1γ作為糖尿病視網膜病變中植物性產物TNTL的治療靶點(影響因子5.111):
視網膜內皮細胞功能障礙的炎症反應被認為在糖尿病視網膜病變(DR)中起重要作用。到目前為止,抗炎治療作為DR治療獲得了不令人滿意的結果。本研究旨在探索一種草藥配方糖寧通絡(TNTL)作為DR治療中的一種新型抗炎藥。我們觀察到高劑量的TNTL在小鼠I型糖尿病模型中具有降血糖作用,而在非低血糖劑量下它抑制DR發生率。TNTL恢復血-視網膜屏障完整性,抑制視網膜新生血管形成,並減弱視網膜神經節細胞變性。對有或沒有TNTL的高血糖小鼠視網膜組織進行轉錄組學分析,發現炎症視網膜微環境受到顯着抑制。TNTL處理抑制視網膜中的促炎性巨噬細胞,導致內皮細胞遷移失活,內皮細胞單層完整性恢復和防止滲漏。細胞因子陣列分析表明,TNTL可顯着抑制促炎巨噬細胞分泌MIP1γ。TNTL預防內皮功能障礙可能是通過抑制MIP1γCCR1軸介導的。更具體地,TNTL抑制促炎巨噬細胞釋放MIP1γ,其反過來抑制內皮細胞中CCR1相關信號傳導途徑的活化。我們的研究結果表明,TNTL可能是DR的替代治療,也是視網膜微環境中針對DR靶向MIP1γCCR1軸的潛在候選藥物的主要來源。
3、“Science Advance科學進展”的論文發表後,在香港大學李嘉誠醫學院的媒體採訪中,已有數家新聞報紙報道了這一發現,認為該發現為肥胖和糖尿病提供了新的治療靶點和新的藥物治療的可能性。
二、香港大學研究發現與進度情況:
TNTL derivatives TNTL化合物系列
1、Aim of study: to screen isolated and synthetic compound from TNTL which can up-regulate SBP2 expression in macrophage and/or can re-skew M1 macrophage potentially M2 phenotype.
目的:考察TNTL化合物系列是否能夠升高巨噬細胞SBP2表達,或者使其從促炎性表型轉變為抗炎性表型。
2、Methods: BMDM was primarily isolated from C57/BL/J mice and cultured into M1-like macrophage (ATM-like phenotype). Chemical derivatives of TJ (FT000-FT040) was given to the M1-like macrophage for 24 hours at the doses of 10μM. Cell were then collected and RNA extracted to proceed for qPCR analysis on related gene expression.
方法:我們在原代巨噬細胞中用藥物處理,用流式細胞術考察其M1/M2表型變化,再用qPCR檢測其SBP2及促炎性和抗炎性細胞因子的表達。
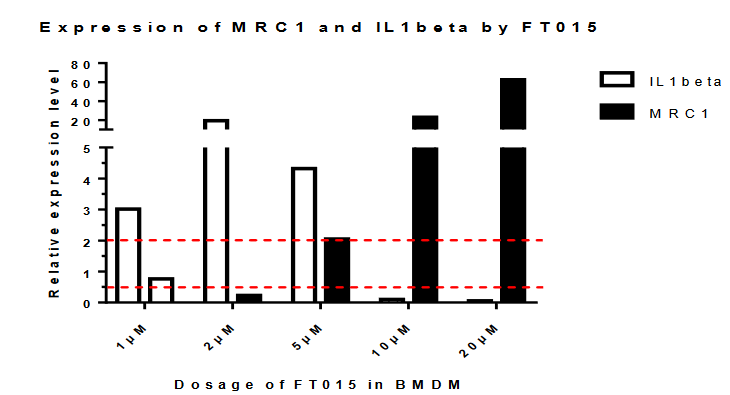
3、Findings: In the previous stage, the FT009 and 030 were found to have significant increase in SBP2 expression (Fig.1) , while FT013,015,016,018,019,025,033 were found to have the potential effect of skewing M1-like macrophage into M2-like phenotype (Fig.2).
之前的研究表明,有幾個化合物(FT013,015,016,018,019,025,033)是有潛力的。但由於巨噬細胞體外模型具有一定的不穩定性,我們將這些化合物進行重複實驗。
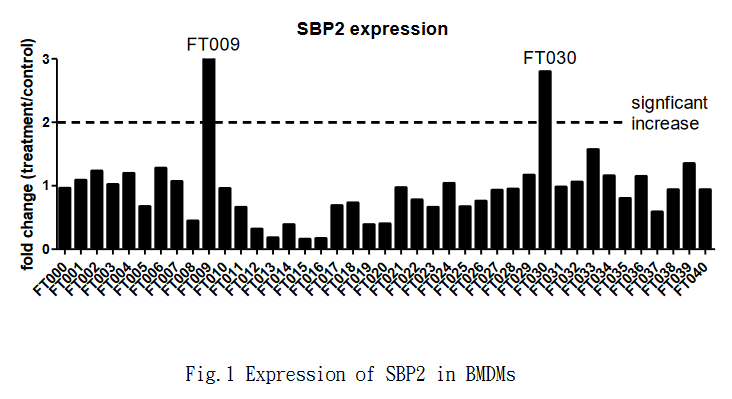
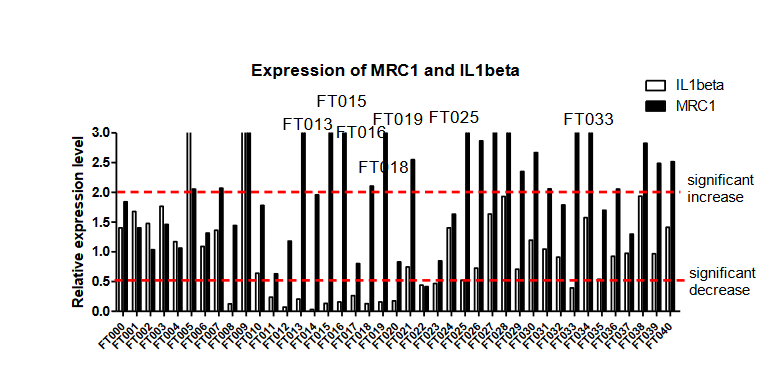
Fig.2 Expression of MRC1 and IL1beta in BMDM
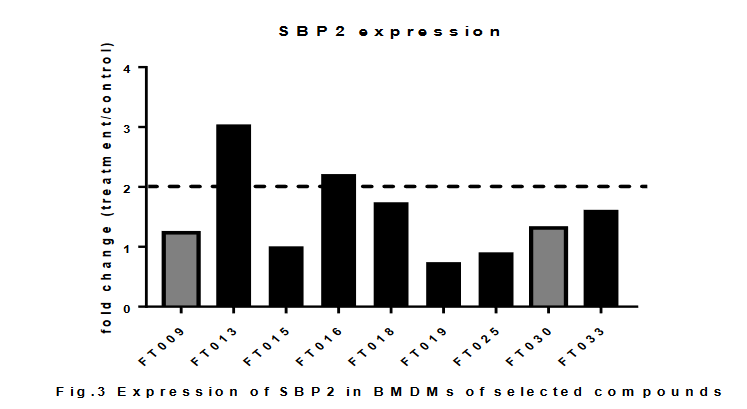
Here, we continue to validate the above-mentioned compounds on their effects in SBP2 elevation and macrophage phenotypic re-skewing. Asshown in Fig.3, the FT009 and FT030 compounds that were previously mentioned to have induced expression of SBP2, did not shown significant changes in the repeated experiment. However, another compound FT013 and FT016 had significant fold change. The inconsistency in the experiments suggested the non-stable effect of the compounds, or a false-positive effect.
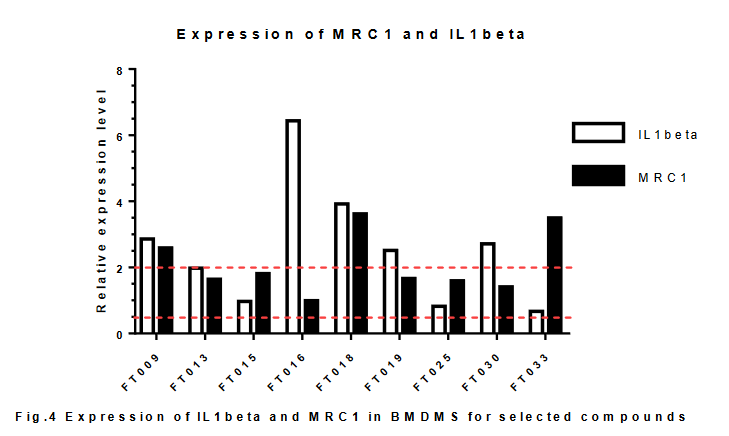
重複的結果與上次結果不太一致。發現只有013和016顯着升高SBP2.
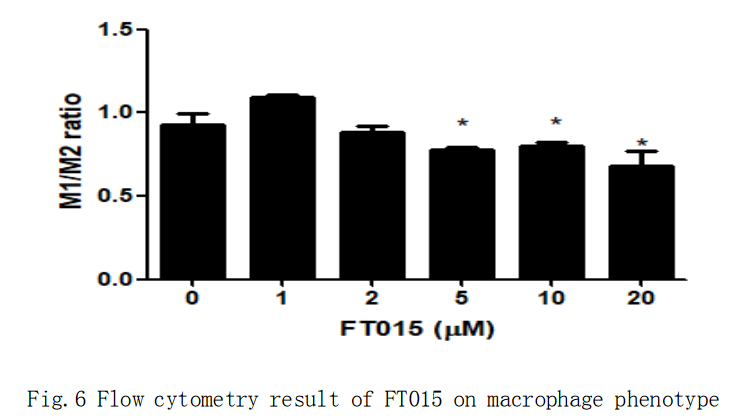
For the M1 to M2 re-kewing, the compounds that were shown effective were repeatedly tested. None of them were oberved consistent result in the first experiment in qPCR of IL1beta and MRC1 gene. The M1/M2 ratio was calculated using the result from the flow cytometry, where M1 population was marked a F4/80+ CD86+, and M2 population was marked as F4/80+ CD206+. As shown in fig.5, the M1/M2 was reduced very dramatically in FT015.
同時,只有015能夠改變M細胞表型。由於升高SBP2並非改變巨噬細胞唯一條件,同時,源化合物FT000並無在體內外證實SBP2為其治療靶點,我們以與疾病更相關的巨噬細胞表型為篩選標準,選定015進行重複。
To further validate the result in the last part, different dosage of FT015 compound was added to the BMDMs. The flow cytometry showed significant M1/M2 ratio improvement in 1μM, 2μM and 5μM, while the 10μM and 20μM group showed lightly decrease but not significant changes. This i inconsitent with the flow result performed last time, with 10μM as the only dosage for FT015. The samples were undergone gene expression analysis, and the IL1beta and MRC1 gene expression pattern suggested the successful induction of re-kewing M1 to M2 by FT015at 10μM and 20μM.
重複結果表明,015的確對於巨噬細胞表型變化有作用,並且有劑量依賴作用。因此,我們建議015可以作為下一個階段考察的對象。
4、Conclusion: We find that FT015 may be the potential compound to change macrophage phenotype from M1 to M2, which are potentially contributing to the anti-diabetic effect.
結論:FT015可能是一個能夠改變巨噬細胞表型的化合物,並潛在地具有抗糖尿病作用。相關作用須在動物實驗上具體驗證。
三、進展總結
1、基於糖寧通絡膠囊臨牀有效性,研究團隊設計了前期課題,驗證了對糖尿病眼病和I、II型糖尿病的藥理作用,探討了相關的作用機制。已有兩篇高水平的研究論文被國際著名雜誌接受,正式出版。研究發現糖寧通絡能減肥和減輕糖尿病耐藥性,對其降血糖、降血脂、改善胰島素抵抗等作用進行了深入的考察,並對其作用機制進行了一系列的深入研究,進一步發現糖寧通絡膠囊改善II型糖尿病的新機制剛好就是脂肪組織上的肥大細胞,脂肪組織中的MII細胞逐漸轉變為促炎性的MI細胞,是糖尿病發病及難治性的病理學基礎之一,糖寧通絡膠囊可以使MI轉化為MII,抗糖尿病的作用機制可能來自於MII對脂肪組織的微環境的改善,最後找到了糖寧通絡膠囊的分子靶點,即SBP2,該蛋白是一個硒結合蛋白,已經報導在糖尿病患者中表達下調,但意義不明,研究證明了糖寧通絡膠囊通過SBP2達到減肥降糖的作用,雖然SBP2是一種硒結合蛋白,但單純補充硒並不能達到同樣的目的,説明糖寧通絡這一苗藥複方具有不可替代性,也是通過臨牀有效的傳統複方找到的新的作用機制和作用靶點,在世界上為肥胖和糖尿病提供了一個新的作用靶點,具有重大意義,值得進一步深入研究和擴大研究範圍。如果説,前期認為糖寧通絡作用於肥大細胞或硒結合蛋白還是一種猜測或假設的話,而當論文正式出版時,各種最新的研究技術已經證明了糖寧通絡作用於肥大細胞或硒結合蛋白與肥胖和糖尿病的關係,是一種因果關係。
2、在本階段,瞄準糖寧通絡膠囊的分子靶點,開始了對從糖寧通絡浸膏中分離純化得到的原型化合物FT000的40個衍生物進行了活性篩選驗證,之前的研究表明,FT013,015,016,018,019,025,033有潛力,重複驗證僅有FT013,016明顯升高SBP2, 但015F可改變巨噬細胞表型,進一步驗證説明T015可能是一個能夠改變巨噬細胞表型的化合物,並潛在地具有抗糖尿病作用。
3、相關作用將在接下來的動物實驗上進一步驗證。
4、基於新發現SBP2靶點和糖寧通絡原方及衍生物的研究結果,已經申請了“一種治療胰島素抵抗的靶點及其應用”的發明專利,並於2019年8月13日獲得受理通知書。
四、後期計劃
在接下來的研究中,香港大學將進一步利用體內外模型,比較研究糖寧通絡,其提取物和其FT013、FT016和FT015衍生物的作用特點和作用機制。



