國藥股份再度觸及漲停!旗下疫苗進入臨牀三期,首次展開跨國試驗
來源:同花順金融研究中心
6月23日報道,國藥集團中國生物新冠滅活疫苗國際臨牀(Ⅲ期)阿拉伯聯合酋長國啟動儀式在中國北京、武漢、阿聯酋阿布扎比三地,以視頻會議方式同步舉行,阿聯酋衞生部長向國藥集團中國生物頒發了臨牀試驗批准文件。
儀式上,中阿雙方現場簽署了相關臨牀合作協議,標誌着全球首個新冠滅活疫苗國際臨牀試驗(Ⅲ期)正式啟動。這是中國原創的疫苗首次在國際上開展Ⅲ期臨牀研究,是中國新冠疫苗在海外開展的第一個臨牀試驗,開啟了新冠疫苗國際合作新篇章。
全球加快疫苗研發我國明顯領先
從6月12日開始,由北京新發地出現新增疫情病例,北京多數地區管控級別升級,疫情管控仍不可掉以輕心;從全球發展趨勢來看,形式非常嚴峻。全球疫情的持續更加凸顯了疫情研發的急迫性,從防護角度看,疫苗是阻擋疾病傳播的根本措施。
據WHO的統計數據,截至6月18日,全球有13個疫苗在臨牀階段,128個疫苗在臨牀前研究階段,路徑包括滅活疫苗、病毒載體疫苗、蛋白重組亞單位疫苗、核酸疫苗、減毒活疫苗等多條路徑。其中,我國多條路徑同時展開研發,進度最快的達到Ⅱ期揭盲階段,從進入臨牀數量來講,我國明顯領先。綜合來看,在未進行到3期臨牀前,沒有十足的把握判斷哪一種疫苗,哪一路徑的疫苗會研製成功,從目前已有的數據看,滅活疫苗在I/II顯示出良好的安全性和免疫原性,滅活疫苗是成熟的技術路徑,後續推進到III期,到最後獲批的前景良好,成功概率大;康希諾生物表示其Ad5載體COVID-19疫苗值得進一步研究,也存在研發成功的一定機率;核酸疫苗以Moderna為代表,其mRNA-1273疫苗的部分受試者顯示出產生中和抗體滴度方面優異的表現,潛力亦值得期待。
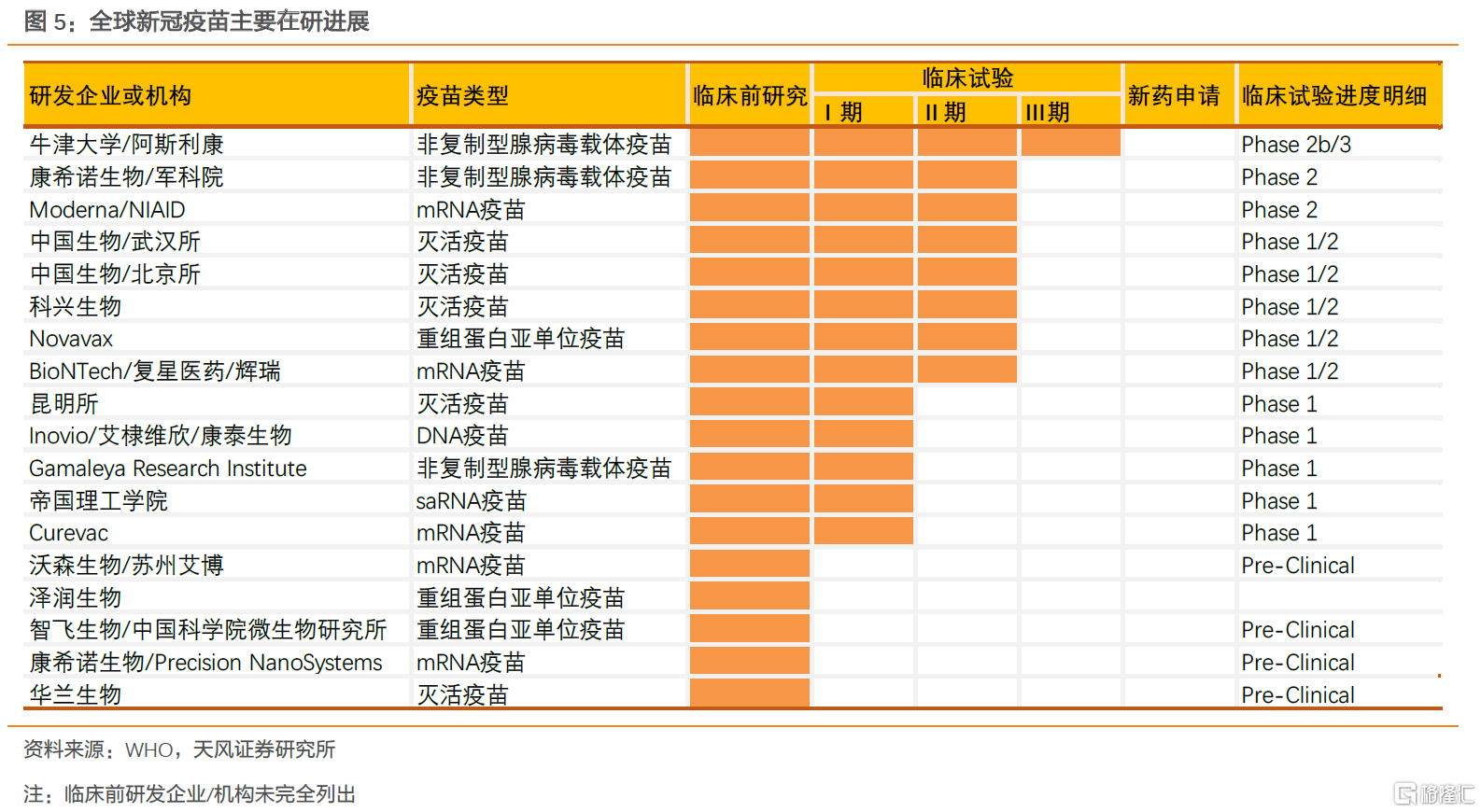
一期臨牀試驗由於是首次在人體進行藥物實驗,因此主要目的有兩個,一是對藥物的安全性和及在人體的耐受性進行研究二期臨牀試驗重點在於藥物的安全性和療效,並進行一些對照性試驗,以確定三期臨牀試驗的給藥劑量和方案,相對的試驗人數規模較小。三期臨牀則是大規模的試驗,一般需要幾百上千人,最低的病例數(試驗組)也需要300例以上。三期臨牀重點在檢測疫苗是否能在大規模人羣中有效,是疫苗研發的決定性步驟。
指數震盪調整疫苗板塊繼續表現
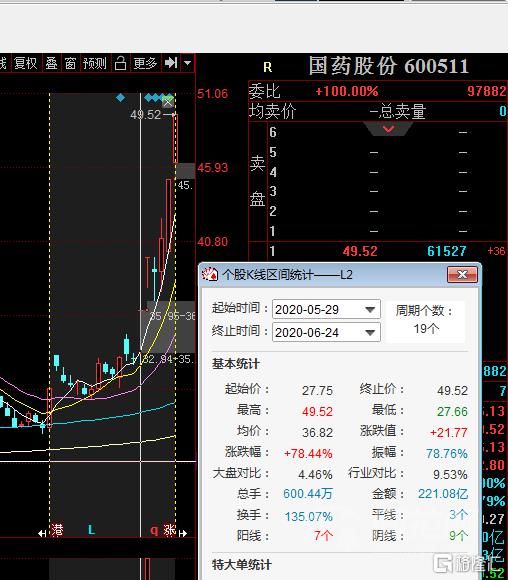
今日三大指數高開後維持橫盤震盪,疫苗概念股繼續表現。截止發文,國藥股份漲停報49.52元/股,成交額逾25億元,值得注意的是,該股自底部啟動以來,已收出80%漲幅。
天風證券研報表示,在新冠疫情下,疫苗研發備受市場關注,挑戰與機遇並存,針對新冠肺炎的疫苗研發,傳統路徑和新疫苗路徑均在同步展開,企業迎來了良好的新技術佈局時點,當前時點是行業新技術儲備的機會,有利於奠定長期發展。此次疫情對於居民的健康意識的提升,對疫苗的認知度有望大幅提升,從中長期看有利於非規劃疫苗接種率的持續提升;此外,新冠肺炎疫苗開發受到重視,研發如火如荼,相關公司加大研發力度和技術引進,有利於推動行業技術平台建設,推動行業創新發展。
Follow us
Find us on
Facebook,
Twitter ,
Instagram, and
YouTube or frequent updates on all things investing.Have a financial topic you would like to discuss? Head over to the
uSMART Community to share your thoughts and insights about the market! Click the picture below to download and explore uSMART app!

Disclaimers
uSmart Securities Limited (“uSmart”) is based on its internal research and public third party information in preparation of this article. Although uSmart uses its best endeavours to ensure the content of this article is accurate, uSmart does not guarantee the accuracy, timeliness or completeness of the information of this article and is not responsible for any views/opinions/comments in this article. Opinions, forecasts and estimations reflect uSmart’s assessment as of the date of this article and are subject to change. uSmart has no obligation to notify you or anyone of any such changes. You must make independent analysis and judgment on any matters involved in this article. uSmart and any directors, officers, employees or agents of uSmart will not be liable for any loss or damage suffered by any person in reliance on any representation or omission in the content of this article. The content of the article is for reference only and does not constitute any offer, solicitation, recommendation, opinion or guarantee of any securities, virtual assets, financial products or instruments. Regulatory authorities may restrict the trading of virtual asset-related ETFs to only investors who meet specified requirements. Any calculations or images in the article are for illustrative purposes only.
Investment involves risks and the value and income from securities may rise or fall. Past performance is not indicative of future performance. Please carefully consider your personal risk tolerance, and consult independent professional advice if necessary.





