三生製藥(01530.HK):重磅品種賽普汀獲批上市,估值具有吸引力,維持"買入"評級
機構:光大證券
評級:買入
事件:公司公告1)三生國健自主研發的注射用伊尼妥單抗(商品名:賽普汀)獲批上市,獲批首個適應症為和化療聯合用於治療HER2 陽性的轉移性乳腺癌。2)建議贖回於2022年到期的可換股債券,新發行總額 3.2 億歐元的可換股債券,換股價13.18港元,較當日收盤價溢價 25%。
點評:
重磅品種賽普汀獲批上市,有望貢獻新增量。伊尼妥單抗治療 HER2 陽性的轉移性乳腺癌 III 期臨牀入組 341 例患者,在主要療效指標方面,試驗組中位 PFS 為 39.1 周(95%CI,31.9-48.1),對照組中位 PFS 為 14.1 周(95%CI,8.4-20.9),顯著延長患者腫瘤無進展生存期。根據國家癌症中心數據,2015 年乳腺癌新發患者超 30 萬,其中 20~25%為 HER2 陽性患者,預期 HER2 單抗藥物市場規模有望於 2023 年達到 94 億元。賽普汀是公司重磅研發落地的新開始,目前該品種進度靠前,HER2 單抗市場競爭相對温和,我們預計峯值有望接近20億元,成為重要增量貢獻。
核心品種益賽普仍佔市場主導地位,特比澳銷售持續強勁。益賽普 19 年市場份額約為 60.9%,受競品納入醫保及疫情影響,預計 20 年益賽普增速略有下滑,好於悲觀預期;預充式水針劑型已申請生產,有望增加患者便利性和依從性。考慮到益賽普滲透率仍有較大空間,未來有望維持温和增長。其他品種中,預計特比澳20年保持雙位數增速,新拓適應症有望提供新增量;促紅素中標價企穩後 20年有望維持雙位數增長;蔓迪療效明確、增長強勁,預計於 24年超10億;糖尿病板塊隨着產品逐漸擴充長期有望貢獻增量。
三生國健科創板上市在即,在研管線不斷豐富。公司旗下三生國健即將於科創板上市,擬募集 32 億元加大研發活動與項目投入力度,在研產品陣容強大、梯隊完善。研發進度方面:益賽普預充式注射劑有望於20 年獲批上市;抗 CD20 單抗與美羅華頭對頭比較的 I 期臨牀完成。早期品種中抗 PD1 單抗獲FDA新藥臨牀批件,I期入組順利;IL17 獲中國臨牀批件並開始 I期入組;眼用VEGF 取得多個臨牀批件。
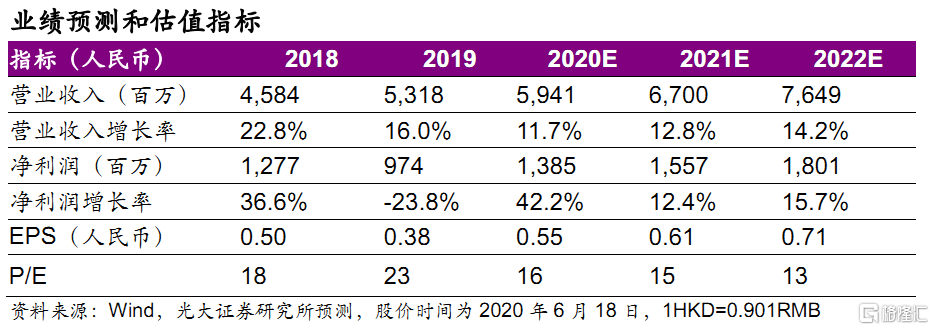
盈利預測與估值。公司是生物藥龍頭企業,我們維持 20~22 年 EPS 為0.55/0.61/0.71 元,同比分別42.4%/+12.4%/15.7%,對應20~22年PE為16/15/13x,估值具有吸引力,維持“買入”評級。
風險提示:研發進度不及預期,銷售不及預期。
Follow us
Find us on
Facebook,
Twitter ,
Instagram, and
YouTube or frequent updates on all things investing.Have a financial topic you would like to discuss? Head over to the
uSMART Community to share your thoughts and insights about the market! Click the picture below to download and explore uSMART app!

Disclaimers
uSmart Securities Limited (“uSmart”) is based on its internal research and public third party information in preparation of this article. Although uSmart uses its best endeavours to ensure the content of this article is accurate, uSmart does not guarantee the accuracy, timeliness or completeness of the information of this article and is not responsible for any views/opinions/comments in this article. Opinions, forecasts and estimations reflect uSmart’s assessment as of the date of this article and are subject to change. uSmart has no obligation to notify you or anyone of any such changes. You must make independent analysis and judgment on any matters involved in this article. uSmart and any directors, officers, employees or agents of uSmart will not be liable for any loss or damage suffered by any person in reliance on any representation or omission in the content of this article. The content of the article is for reference only and does not constitute any offer, solicitation, recommendation, opinion or guarantee of any securities, virtual assets, financial products or instruments. Regulatory authorities may restrict the trading of virtual asset-related ETFs to only investors who meet specified requirements. Any calculations or images in the article are for illustrative purposes only.
Investment involves risks and the value and income from securities may rise or fall. Past performance is not indicative of future performance. Please carefully consider your personal risk tolerance, and consult independent professional advice if necessary.




